Alleviating the burden of PACG treatment
- Technical Articles
Primary angle-closure glaucoma (PACG) represents a significant cause of blindness, affecting more than 17 million people worldwide. The different techniques of minimally invasive glaucoma surgery (MIGS) that have been developed for the management of open-angle glaucoma with a gonioscopic ab interno approach, are not applicable in case of a shallow anterior chamber or when a narrow angle prevents access to the corneoscleral trabecular meshwork (trabecular stents) or in case of cyclodialysis (supraciliary devices). The cilioscleral inter-positioning device is a novel minimally invasive implant placed ab externo between the sclera and the ciliary body to increase uveoscleral outflow without entering the chamber or creating subconjunctival filtration. This unique approach to lower IOP represents a new, efficient, and safe surgical option to reduce intraocular pressure for patients with PACG.
What is angle-closure glaucoma?
Primary angle-closure glaucoma (PACG), also called narrow-angle glaucoma, is a chronic disorder, usually caused by pupillary block, which is characterized by increased resistance towards the aqueous humor flow through the iris-lens channel from the posterior to anterior chamber, resulting in increased intraocular pressure (IOP). A less common cause of PACG is angle crowding, which is due to compression of the iris between the trabecular meshwork and another anatomical structure such as the iris. Plateau iris syndrome, defined as a persistently narrow angle capable of closure in spite of a patent iridotomy, represents the most common form of iris crowding and is present in about 30% of the patients suspected of angle closure [1]. Secondary ACG can be caused by a myriad of ocular diseases such as neovascularization or uveitis and can be either unilateral or bilateral. Both primary and secondary forms of ACG can cause an increase of the IOP usually exceeding 40 mmHg and may rise to as high as 80 mmHg.
Although primary open-angle glaucoma is more common, PACG are usually more severe and more likely to result in irreversible blindness [2], therefore early and effective interventions are important. Moreover, chronic PACG can also create acute and punctual episodes of angle closure, which can significantly reduce the visual field as well as the corneal endothelial density [3, 4] and which require immediate action.
What is the prevalence of patients with ACG and what are the risk factors?
The prevalence of patients with primary ACG aged 40-80 is estimated at 0.50% and the number of people with ACG worldwide will increase to 32 million in 2040 [5]. The highest prevalence is in Asia with 1.09%, representing 75% of the number of patients with ACG worldwide [5]. Overall, primary ACG represents a large cause of blindness worldwide, [6] which combined with its severity, gives ACG a high disease burden.
Numerous risk factors have been identified for ACG including hyperopia, family history of angle closure, advancing age, female sex, Asian or Inuit descent, shallow anterior chamber depth, shorter axial length and thicker lens [7].
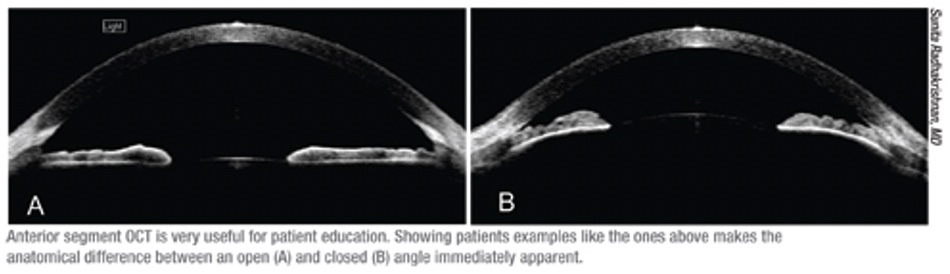
Today, quantitative anatomical measurements with anterior segment OCT can identify patients at risk of progression from primary angle closure suspects (PACS) to primary angle closure (PAC) and to select an optimal surgical solution [8].
What are the currently available treatment options for patients with ACG?
There is no curative medical therapy for ACG, so the main objectives for disease management are to reverse or prevent the angle closure process, control intraocular pressure elevation, and prevent damage to the optic nerve.
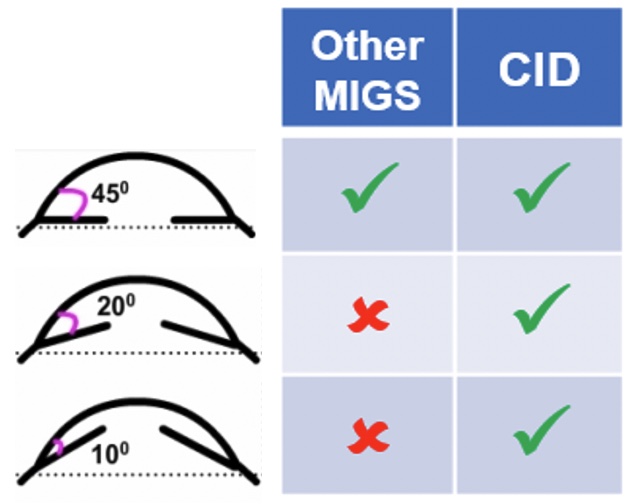
Topical hypotensive medications are usually used as first-line treatment to reduce intraocular pressure of patients with acute ACG. However, medications present several disadvantages such as patient non-compliance [9], incompatibility with self-administration [10], side effects [11], and limited access for financial reasons [12]. Thermal or laser peripheral iridotomy represents an effective technique to open the drainage pathway [6]. However, numerous complications can occur following this procedure, including increased IOP, laser burn to the cornea, lens, or retina, late-onset corneal edema, development of posterior synechiae, hyphemia, iritis, and development of visual disturbances [6]. Clear lens extraction with intraocular lens implantation have also been demonstrated as effective on patients with PACG and are can now be considered as first-line treatment to widen the angle and to decrease the risk of acute angle closure [6]. Filtration surgery can be considered if the optic neuropathy is progressing and IOP is at a level believed to be contributing to the progression [6]. However, ab interno gonioscopic approach incisional surgery with or without implantation of drainage devices available for open-angle glaucoma (MIGS) is not adapted for patients with PACG (Figure 1). This is due to the difference of pathophysiology and underlying causes between both types of glaucoma.
How can the new Cilioscleral Inter-positioning Device overcome these limitations?
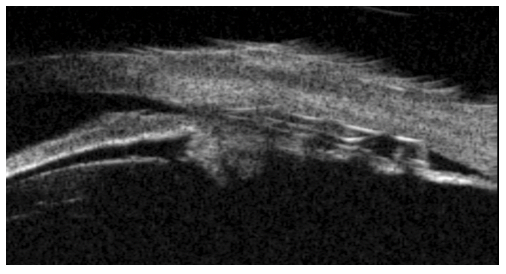
Developed by CILIATECH, the cilioscleral inter-positioning device (CID) is a novel, minimally invasive implant designed to lower IOP of glaucomatous patients without entering the anterior chamber and without bleb. This non-resorbable, 26% hydrophilic acrylic implant has a general size of 6 x 4 x 0.4 mm and can be inserted ab externo behind the limbus in the supraciliary space through two scleral incisions, following the cilioscleral interposition surgical technique (Figure 2). This unique implant location increases the natural uveoscleral pathway while leaving the anterior chamber fully intact, and therefore theoretically is not limited by the anterior chamber and angle conformation. So, unlike other techniques of incisional surgeries and MIGS devices that are only designed for open-angle glaucoma, CID can also be implanted in patients with ACG. Results of clinical trials conducted on 26 patients with ACG showed a IOP reduction of ⁓40% at 12 months as well as a decrease in the burden of use of hypotensive medications from 1.5 to 0.0, and this without accessing the anterior chamber and without removing the lens.
Moreover, very few and very mild complications or adverse events (and especially no angle modification and no device movement) were reported during the studies. Finally, no IOP change and no device displacement were observed when a cataract surgery was performed after CID implantation. Overall, the current clinical data suggest that CID represents a safe and efficient first-line treatment for patients with ACG, and that the cilioscleral approach is “angle agnostic”.

References
- Kumar RS, Baskaran M, Chew PTK, Friedman DS, Handa S, Lavanya R, Sakata LM, Wong H-T, Aung T (2008) Prevalence of plateau iris in primary angle closure suspects an ultrasound biomicroscopy study. Ophthalmology 115:430–434. https://doi.org/10.1016/j.ophtha.2007.07.026
- George R, Panda S, Vijaya L (2022) Blindness in glaucoma: primary open-angle glaucoma versus primary angle-closure glaucoma—a meta-analysis. Eye 36:2099–2105. https://doi.org/10.1038/s41433-021-01802-9
- Bigar F, Witmer R (1982) Corneal endothelial changes in primary acute angle-closure glaucoma. Ophthalmology 89:596–599. https://doi.org/10.1016/s0161-6420(82)34744-2
- Douglas GR, Drance SM, Schulzer M (1975) The visual field and nerve head in angle-closure glaucoma. A comparison of the effects of acute and chronic angle closure. Arch Ophthalmol 93:409–411. https://doi.org/10.1001/archopht.1975.01010020423004
- Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121:2081–2090. https://doi.org/10.1016/j.ophtha.2014.05.013
- (2020) Primary Angle-Closure Disease PPP 2020. In: American Academy of Ophthalmology. https://www.aao.org/education/preferred-practice-pattern/primary-angle-closure-disease-ppp. Accessed 6 Sep 2023
- Wright C, Tawfik MA, Waisbourd M, Katz LJ (2016) Primary angle-closure glaucoma: an update. Acta Ophthalmol 94:217–225. https://doi.org/10.1111/aos.12784
- Xu BY, Friedman DS, Foster PJ, Jiang Y, Porporato N, Pardeshi AA, Jiang Y, Munoz B, Aung T, He M (2022) Ocular Biometric Risk Factors for Progression of Primary Angle Closure Disease: The Zhongshan Angle Closure Prevention Trial. Ophthalmology 129:267–275. https://doi.org/10.1016/j.ophtha.2021.10.003
- Tsai JC (2009) A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology 116:S30-36. https://doi.org/10.1016/j.ophtha.2009.06.024
- Brown MM, Brown GC, Spaeth GL (1984) Improper topical self-administration of ocular medication among patients with glaucoma. Can J Ophthalmol 19:2–5
- Taylor SA, Galbraith SM, Mills RP (2002) Causes of non-compliance with drug regimens in glaucoma patients: a qualitative study. J Ocul Pharmacol Ther 18:401–409. https://doi.org/10.1089/10807680260362687
- Zhao PY, Rahmathullah R, Stagg BC, Almobarak F, Edward DP, Robin AL, Stein JD (2018) A Worldwide Price Comparison of Glaucoma Medications, Laser Trabeculoplasty, and Trabeculectomy Surgery. JAMA Ophthalmology 136:1271–1279. https://doi.org/10.1001/jamaophthalmol.2018.3672